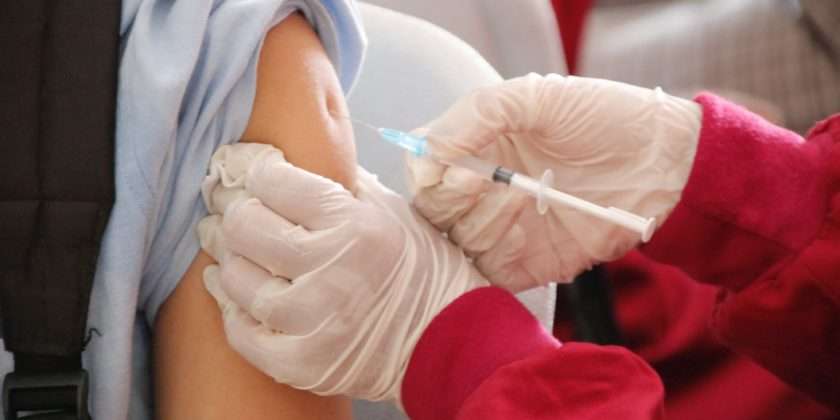
The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least two months after completing primary or booster vaccination1. This post “COVID-19 Bivalent Vaccine Boosters:9/22/22 “seeks to provide current and relevant information from a trusted source.
The virus that causes COVID-19 changes over time. Keep your protection against COVID-19 up to date by getting a bivalent COVID-19 vaccine booster dose. Why is this important? See the COVID19 Stats below2:
Covid 19 Stats (10-3-22 @2:40 PM ET)
The bivalent COVID-19 vaccines include a component of the original virus strain to provide broad protection against COVID-19 and a component of the omicron variant to provide better protection against COVID-19 caused by the omicron variant. These are called bivalent COVID-19 vaccines because they contain these two components. A bivalent COVID-19 vaccine may also be referred to as “updated” COVID-19 vaccine booster dose.
COVID-19 vaccines can help protect against severe illness, hospitalization and death from COVID-19. As the virus changes and your immunity naturally decreases over time, you may lose some of that protection.
Search in your zip code to find a location where a bivalent COVID-19 vaccine booster dose is available near you.
What bivalent COVID-19 vaccine boosters has FDA authorized?
Video: What data support the use of the updated COVID-19 boosters? [1:31]
The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least two months after completing primary or booster vaccination.
The Moderna COVID-19 Vaccine, Bivalent is authorized for use as single booster dose in individuals 18 years of age and older.
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is authorized for use as a single booster dose in individuals 12 years of age and older.
Am I eligible for an updated (bivalent) COVID-19 vaccine booster?
Eligibility for a booster depends on:
- Your age
- When you completed your primary vaccination
OR - When received your most recent booster dose of a monovalent COVID-19 vaccine
Use this CDC online tool to find out when you can get a COVID-19 vaccine booster.
If eligible, consider getting a bivalent COVID-19 vaccine booster dose.
Video: What is a bivalent vaccine? [1:20]
A single booster dose with an updated bivalent COVID-19 vaccine provides broad protection against COVID-19 and is expected to provide better protection against COVID-19 caused by the currently circulating Omicron variant.
If you are eligible for an updated bivalent COVID-19 vaccine booster, the updated booster dose that you receive does not need to be from the same manufacturer that made the vaccine that you received for your primary vaccination or previous booster with a monovalent COVID-19 vaccine.
Click this link to get free Health and Wealth information to improve your life. Play the free “Slow Roll Through Civil Rights” Game found on the Jay Harold website. Enjoyed this post? Share it and read more here.
Bibliography
- https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-bivalent-vaccine-boosters
- https://covid.cdc.gov/covid-data-tracker/#datatracker-home