Cytokine release syndrome (a severe and possibly life-threatening reaction) that occurs in adults and children 2 years of age or older after receiving certain immunotherapy infusions. Cytokine release syndrome (CRS) can cause severe illness and death in some people with COVID-191. However, scientists are learning how to treat CRS in these individuals. Researchers in China found that tocilizumab helped treat CRS in 21 people with SARS-CoV-2, reducing their fever in 24 hours. ACTEMRA is indicated for the treatment of life-threatening cytokine release syndrome. This post ”Cytokine release syndrome: COVID19” seeks to provide current and relevant information from a trusted source.
Tocilizumab2 injection is used alone or in combination with other medications to relieve the symptoms of certain types of arthritis and other conditions including:
-
- rheumatoid arthritis (a condition in which the body attacks its own joints, causing pain, swelling, and loss of function) in people who have not been helped by other disease-modifying antirheumatic drugs (DMARDs),
- giant cell arteritis (a condition that causes swelling of blood vessels, especially in the scalp and head), polyarticular juvenile idiopathic arthritis (PJIA; a type of childhood arthritis that affects five or more joints during the first six months of the condition, causing pain, swelling, and loss of function) in children 2 years of age or older.
- systemic juvenile idiopathic arthritis (SJIA; a condition in children that causes inflammation in different areas of the body, causing fever, joint pain and swelling, loss of function, and delays in growth and development) in children 2 years of age or older.
- cytokine release syndrome (a severe and possibly life-threatening reaction) that occurs in adults and children 2 years of age or older after receiving certain immunotherapy infusions.
Tocilizumab injection is in a class of medications called interleukin-6 (IL-6) receptor inhibitors. It works by blocking the activity of interleukin-6, a substance in the body that causes inflammation.
How should this medicine be used?
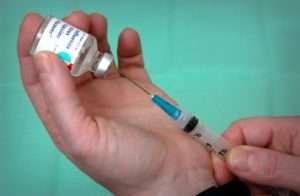
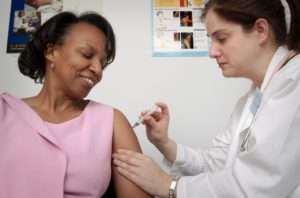
Tocilizumab injection comes as a solution (liquid) to be injected intravenously (into a vein) in your arm by a doctor or nurse in a medical office or hospital outpatient clinic or as a prefilled syringe to inject subcutaneously (under the skin) by yourself at home. When tocilizumab is given intravenously to treat rheumatoid arthritis or polyarticular juvenile idiopathic arthritis, it is usually given once every 4 weeks. When tocilizumab is given intravenously to treat systemic juvenile idiopathic arthritis, it is usually given once every 1 or 2 weeks. When tocilizumab is given intravenously to treat cytokine release syndrome, it is usually given once, but up to 3 additional doses may be given at least 8 hours apart. It will take about 1 hour for you to receive your dose of tocilizumab injection intravenously. When tocilizumab is given subcutaneously to treat rheumatoid arthritis or giant cell arteritis, it is usually given once weekly or once every other week.
Other uses for this medicine
This medication may be prescribed for other uses; ask your doctor or pharmacist for more information.
What special precautions should I follow?
Before receiving tocilizumab injection,
- tell your doctor and pharmacist if you are allergic to tocilizumab, any other medications, or any of the ingredients in tocilizumab injection. Ask your pharmacist or check the Medication Guide for a list of the ingredients.
Cancer?
- tell your doctor if you have or have ever had cancer; diverticulitis (small pouches in the lining of the large intestine that can become inflamed); ulcers in your stomach or intestines; high cholesterol and triglycerides; any condition that affects the nervous system such as multiple sclerosis (MS; a disease in which the nerves do not function properly and people may experience weakness, numbness, loss of muscle coordination and problems with vision, speech, and bladder control) or chronic inflammatory demyelinating polyneuropathy (CIDP; a disorder of the immune and nervous systems); or liver disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breastfeeding. If you become pregnant while receiving tocilizumab injection, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are receiving tocilizumab injection.
- ask your doctor whether you should receive any vaccinations before you begin your treatment with tocilizumab injection. If possible, all vaccinations for children should be brought up to date before beginning treatment. Do not have any vaccinations during your treatment without talking to your doctor.
What special dietary instructions should I follow?
Unless your doctor tells you otherwise, continue your normal diet.
Tocilizumab injection may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- headache
- runny nose or sneezing
- redness, itching, pain, or swelling in the place where tocilizumab was injected
Some side effects can be serious. If you experience any of these symptoms or those listed in the IMPORTANT WARNING section, call your doctor immediately or get emergency medical treatment:
- rash
- flushing
- hives
- itching
- swelling of the eyes, face, lips, tongue, throat, arms, hands, feet, ankles, or lower legs
- difficulty breathing or swallowing

- chest pain
- dizziness or fainting
- fever, ongoing stomach-area pain, or change in bowel habits
- yellow eyes or skin; right upper abdominal pain; unexplained bruising or bleeding; loss of appetite; confusion; yellow or brown-colored urine; or pale stools
Tocilizumab may increase the risk of developing certain types of cancer. Talk to your doctor about the risks of receiving this medication. Tocilizumab injection may cause other side effects. Call your doctor if you have any unusual problems while receiving this medication.
If you experience a serious side effect, you or your doctor may send a report to the Food and Drug Administration’s (FDA) MedWatch Adverse Event Reporting program online (http://www.fda.gov/Safety/MedWatch) or by phone (1-800-332-1088).
In case of emergency/overdose
In case of overdose, call the poison control helpline at 1-800-222-1222. Information is also available online at https://www.poisonhelp.org/help. If the victim has collapsed, had a seizure, has trouble breathing, or can’t be awakened, immediately call emergency services at 911.
What other information should I know?
Ask your pharmacist any questions you have about tocilizumab injection.
Do not let anyone else use your medication. Ask your pharmacist any questions you have about refilling your prescription.
It is important for you to keep a written list of all of the prescription and nonprescription (over-the-counter) medicines you are taking, as well as any products such as vitamins, minerals, or other dietary supplements. You should bring this list with you each time you visit a doctor or if you are admitted to a hospital. It is also important information to carry with you in case of emergencies.
Brand names3
- Actemra®
Last Revised – 08/15/2019
Proposed Rationale for Use of corticosteroids in Patients with COVID-194
Patients with severe COVID-19 can develop a systemic inflammatory response that can lead to lung injury and multisystem organ dysfunction. It has been proposed that the potent anti-inflammatory effects of corticosteroids might prevent or mitigate these deleterious effects.
Several of these studies demonstrated clinical benefit with the early use of low-dose methylprednisolone, including a more rapid resolution of hypoxia, reduction in the requirement for mechanical ventilation, and a reduction in transfer to the intensive care unit and in-hospital length of stay.
Why is Methylprednisolone prescribed5?
Methylprednisolone injection is used to treat severe allergic reactions. Methylprednisolone injection is used in the management of multiple sclerosis (a disease in which the nerves do not function properly), lupus (a disease in which the body attacks many of its organs), gastrointestinal disease, and certain types of arthritis. Methylprednisolone injection is also used to treat certain conditions that affect the blood, skin, eyes, nervous system, thyroid, kidneys, and lungs. It is sometimes used in combination with other medications to treat symptoms of low corticosteroid levels (lack of certain substances that are usually produced by the body and are needed for normal body functioning). Methylprednisolone injection is in a class of medications called corticosteroids. It works to treat people with low levels of corticosteroids by replacing steroids that are normally produced naturally by the body. It also works to treat other conditions by reducing swelling and redness and by changing the way the immune system works.
How should this medicine be used?
Methylprednisolone injection comes as a powder to be mixed with liquid to be injected intramuscularly (into a muscle) or intravenously (into a vein). It also comes as a suspension
for injection to be injected intramuscularly, intra-articularly (into a joint), or intralesionally (into a lesion). Your dosing schedule will depend on your condition and on how you respond to treatment.
You may receive methylprednisolone injection in a hospital or medical facility, or you may be given the medication to use at home. If you will be using methylprednisolone injection at home, your healthcare provider will show you how to inject the medication. Be sure that you understand these directions, and ask your healthcare provider if you have any questions. Ask your healthcare provider what to do if you have any problems using methylprednisolone injection.
Your doctor may change your dose of methylprednisolone injection during your treatment to be sure that you are always using the lowest dose that works for you. Your doctor may also need to change your dose if you experience unusual stress on your body such as surgery, illness, or infection. Tell your doctor if your symptoms improve or get worse or if you get sick or have any changes in your health during your treatment.
Other uses for this medicine
Methylprednisolone injection is also sometimes used to treat nausea and vomiting from certain types of chemotherapy for cancer and to prevent organ transplant rejection. Talk to your doctor about the risks of using this medication for your condition.
This medication may be prescribed for other uses; ask your doctor or pharmacist for more information.
What special precautions should I follow?
Before receiving methylprednisolone injection,
- tell your doctor and pharmacist if you are allergic to methylprednisolone, any other medications, benzyl alcohol, or any of the ingredients in methylprednisolone injection. Ask your pharmacist for a list of the ingredients.
- tell your doctor and pharmacist what other prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: aminoglutethimide (Cytadren; no longer available in U.S.); amphotericin B (Abelcet, Ambisome, Amphotec); anticoagulants (‘blood thinner
Fungal Infections
- tell your doctor if you have a fungal infection (other than on your skin or nails). Your doctor will probably tell you not to use methylprednisolone injection. Also, tell your doctor if you have idiopathic thrombocytopenic purpura (ITP; an ongoing condition that may cause easy bruising or bleeding due to an abnormally low number of platelets in the blood). Your doctor probably will not give you methylprednisolone intramuscularly, if you have ITP.
- tell your doctor if you have or have ever had tuberculosis (TB: a type of lung infection); cataracts (clouding of the lens of the eye); glaucoma (an eye disease); Cushing’s syndrome (a condition where the body produces too much of the hormone cortisol); diabetes; high blood pressure; heart failure; a recent heart attack; emotional problems, depression or other types of mental illness; myasthenia gravis (a condition in which the muscles become weak); osteoporosis (a condition in which the bones become weak and fragile and can break easily); seizures; ulcers; or liver, kidney, heart, intestinal, or thyroid disease. Also tell your doctor if you have any type of untreated bacterial, parasitic, or viral infection anywhere in your body or a herpes eye infection (a type of infection that causes a sore on the eyelid or eye surface).
Pregnant?

- tell your doctor if you are pregnant, plan to become pregnant, or are breastfeeding. If you become pregnant while receiving methylprednisolone injection, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are receiving methylprednisolone injection.
- do not have any vaccinations (shots to prevent diseases) without talking to your doctor.
- you should know that methylprednisolone injection may decrease your ability to fight infection and may prevent you from developing symptoms if you get an infection. Stay away from people who are sick and wash your hands often while you are using this medication. Be sure to avoid people who have chickenpox or measles. Call your doctor immediately if you think you may have been around someone who had chickenpox or measles.
What special dietary instructions should I follow?
Your doctor may instruct you to follow a low-salt or a diet high in potassium or calcium. Your doctor may also prescribe or recommend a calcium or potassium supplement. Follow these directions carefully.
What side effects can this medication cause?
Methylprednisolone injection may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- headache
- dizziness
- slowed healing of cuts and bruises
- acne
- thin, fragile, or dry skin
- red or purple blotches or lines under the skin
- skin depressions at the injection site

- increased body fat or movement to different areas of your body
- difficulty falling asleep or staying asleep
- inappropriate happiness
- extreme changes in mood changes in personality
- extreme tiredness
- depression
- increased sweating
- muscle weakness
- joint pain
- dizziness
- irregular or absent menstrual periods
- increased appetite
- hiccups
Some side effects can be serious. If you experience any of these symptoms, call your doctor immediately or get emergency medical treatment:
- sore throat, fever, chills, cough, or other signs of infection
- seizures
- vision problems
- swelling of the eyes, face, lips, tongue, throat, arms, hands, feet, ankles, or lower legs
- difficulty breathing or swallowing
- shortness of breath
- sudden weight gain
- rash
- hives
- itching
- confusion
- abnormal skin patches in the mouth, nose, or throat
- numbness, burning or tingling in the face, arms, legs, feet, or hands
More side effects
Methylprednisolone injection may cause children to grow more slowly. Your child’s doctor will watch your child’s growth carefully while your child is using methylprednisolone injection. Talk to your child’s doctor about the risks of giving this medication to your child.
Methylprednisolone injection may increase your risk of developing osteoporosis. Talk to your doctor about the risks of using this medication.
Methylprednisolone injection may cause other side effects. Call your doctor if you have any unusual problems while using this medication.
If you experience a serious side effect, you or your doctor may send a report to the Food and Drug Administration’s (FDA) MedWatch Adverse Event Reporting program online (http://www.fda.gov/Safety/MedWatch) or by phone (1-800-332-1088).
In case of emergency/overdose
In case of overdose, call the poison control helpline at 1-800-222-1222. Information is also available online at https://www.poisonhelp.org/help. If the victim has collapsed, had a seizure, has trouble breathing, or can’t be awakened, immediately call emergency services at 911.
What other information should I know?
Keep all appointments with your doctor and the laboratory. Your doctor will order certain lab tests to check your body’s response to methylprednisolone injection.
Before having any laboratory test, tell your doctor and the laboratory personnel that you are using methylprednisolone injection.
If you are having any skin tests such as allergy or tuberculosis tests, tell the doctor or technician that you are receiving methylprednisolone injection.
Ask your pharmacist any questions you have about methylprednisolone injection. Do not let anyone else use your medication. Ask your pharmacist any questions you have about refilling your prescription.
It is important for you to keep a written list of all of the prescription and nonprescription (over-the-counter) medicines you are taking, as well as any products such as vitamins, minerals, or other dietary supplements. You should bring this list with you each time you visit a doctor or if you are admitted to a hospital. It is also important information to carry with you in case of emergencies.
Brand names
- A-Methapred®
- Depo-Medrol®
- Solu-Medrol®
Last Revised – 05/15/2016
Jay Harold hopes you enjoyed this post, “Cytokine release syndrome: COVID19.” Please share it and read more about Jay Harold here. Please take this advice from Muhammad Ali and give back to others. “Service to others is the rent you pay for your room here on earth.”
Bibliography
- https://www.medicalnewstoday.com/articles/cytokine-release-syndrome#covid-19
- https://medlineplus.gov/druginfo/meds/a611004.html
- https://www.actemrainfo.com/
- https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/
- https://medlineplus.gov/druginfo/meds/a601157.html