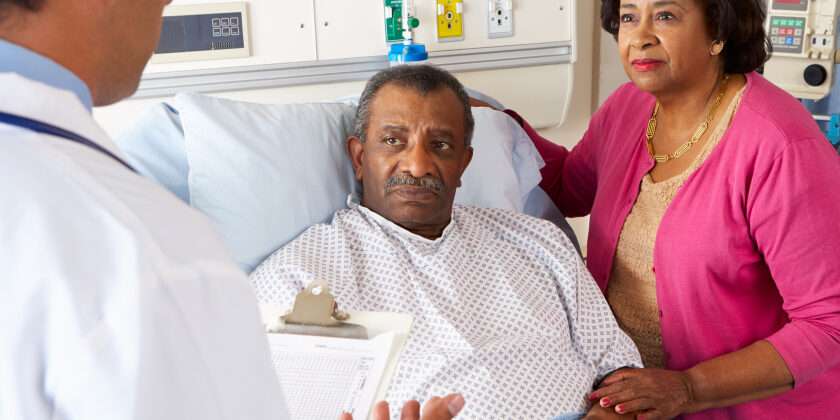
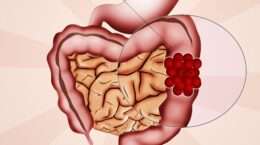
Jay Harold is constantly looking to keep you up to date with the latest healthcare treatments. Jay Harold recently found a new medication used in the treatment of Pancreatic Cancer. Pancreatic Cancer treatment is of particular significant interest to African- Americans. African-Americans have higher rates of pancreatic cancer incidence and mortality than whites or other racial/ethnic groups. There are an estimated 48,960 new cases of Pancreatic Cancer in 2015 according to the National Cancer Institute (NCI). Over 40,500 people are expected to die of Pancreatic Cancer in 2015.
Jay Harold realizes that chemotherapy medications used to treat Cancer are a difficult topic to understand. Awareness of the latest advances in healthcare gives additional tools to your doctor for the benefit of the patient.The advances in the treatment of Cancer also provides hope for the patient and family.
The U.S. Food and Drug Administration recently approved Onivyde (irinotecan liposome injection), in combination with fluorouracil and leucovorin, to treat patients with advanced (metastatic) pancreatic cancer who have been previously treated with gemcitabine-based chemotherapy.
Play the free “Slow Roll Through Civil Rights” Game found on the Jay Harold website. Enjoyed this post? Share it and read more here.